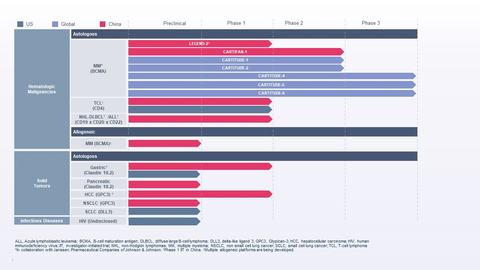
Legend Biotech Reports First Quarter 2022 Financial Results and Recent Highlights
-
The
U.S. Food and Drug Administration (FDA) approved CARVYKTI™ (ciltacabtagene autoleucel; cilta-cel) for the treatment of adults with relapsed or refractory multiple myeloma, marking Legend Biotech’s first product approved by a health authority -
The
European Commission (EC) has granted conditional marketing authorization of CARVYKTI™ for the treatment of adults with relapsed and refractory multiple myeloma -
Legend Biotech engagedErnst & Young LLP (US) as the company’s auditor for the fiscal year endingDecember 31, 2022 -
Legend Biotech achieved milestone payments under its collaboration agreement withJanssen Biotech, Inc. for CARVYKTI™
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220601005384/en/
“The year began with an exciting start as we received the company’s first-ever
First Quarter 2022 Highlights and Recent Events
-
On
February 28, 2022 , theU.S. Food and Drug Administration (FDA) approved CARVYKTI™ for the treatment of adults with relapsed or refractory multiple myeloma who have received four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, marking the company’s first product approved by a health authority. -
On
May 26, 2022 , theEuropean Commission (EC) granted conditional marketing authorization of CARVYKTI™ for the treatment of adults with relapsed and refractory multiple myeloma who have received at least three prior therapies, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD) and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy. -
On
April 21, 2022 , Legend announced the achievement of a$50 million milestone under its collaboration agreement withJanssen Biotech, Inc. (Janssen) for CARVYKTI™.Legend Biotech entered into an exclusive worldwide license and collaboration agreement with Janssen to develop and commercialize CARVYKTI™ inDecember 2017 . -
Legend Biotech engagedErnst & Young LLP , located inthe United States , as the company’s independent, registered public accounting firm for the audits of the Company’s financial statements and internal control over financial reporting for the fiscal year endingDecember 31, 2022 . - CARTITUDE-6 (not yet recruiting; sponsored by the European Myeloma Network), a second Phase 3 trial in frontline multiple myeloma, was posted on clinicaltrials.gov. This Phase 3, randomized, open-label study compares daratumumab, bortezomib, lenalidomide and dexamethasone (DVRd) followed by cilta-cel vs. DVRd followed by autologous stem cell transplant (ASCT) in newly diagnosed, transplant-eligible patients with multiple myeloma.
-
On
May 25, 2022 , The FDA lifted its clinical hold of Legend Biotech’s Phase 1 clinical trial of LB1901, the company’s investigational autologous chimeric antigen receptor T-cell (CAR-T) therapy targeting malignant CD4+ T-cells for the treatment of adults with relapsed or refractory T-cell lymphoma (TCL). -
On
March 23, 2022 ,Legend Biotech was awarded Newcomer of the Year at the tenth annual Foreign Investment Trophy ceremony hosted byFlanders Investment & Trade (FIT) for its joint investment in a state-of-the-art manufacturing facility in Flanders withJanssen Pharmaceutica N.V. -
Legend Biotech made the following management appointments:Lori Macomber , CPA, as Chief Financial Officer;Guowei Fang , Ph.D., as Senior Vice President, Global Head of Research andEarly Development ; andMarc L. Harrison , as Vice President and General Counsel.
Financial Results for First Quarter Ended
Cash and Cash Equivalents, Time Deposits, and Short-Term Investments
As of
Revenue
Revenue for the three months ended
Research and Development Expenses
Research and development expenses for the three months ended
Administrative Expenses
Administrative expenses for the three months ended
Selling and Distribution Expenses
Selling and distribution expenses for the three months ended
Other Income and Gains
Other income and gains for the three months ended
Other Expenses
Other expenses for the three months ended
Finance Costs
Finance costs for the three months ended
Fair Value Gain of Warrant Liability
Fair value gain of warrant liability for the year ended
Loss for the Period
For the three months ended
About
Learn more at www.legendbiotech.com and follow us on Twitter and LinkedIn.
About CARVYKTI™ (Ciltacabtagene autoleucel; cilta-cel)
CARVYKTI™ is a BCMA-directed, genetically modified autologous T-cell immunotherapy, which involves reprogramming a patient’s own T-cells with a transgene encoding a chimeric antigen receptor (CAR) that identifies and eliminates cells that express BCMA. BCMA is primarily expressed on the surface of malignant multiple myeloma B-lineage cells, as well as late-stage B-cells and plasma cells. The CARVYKTI™ CAR protein features two BCMA-targeting single domain antibodies designed to confer high avidity against human BCMA. Upon binding to BCMA-expressing cells, the CAR promotes T-cell activation, expansion, and elimination of target cells.1
In
In
About Multiple Myeloma
Multiple myeloma is an incurable blood cancer that starts in the bone marrow and is characterized by an excessive proliferation of plasma cells.5 In 2022, it is estimated that more than 34,000 people will be diagnosed with multiple myeloma, and more than 12,000 people will die from the disease in the
Cautionary Note Regarding Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to Legend Biotech’s strategies and objectives; statements relating to CARVYKTI™, including Legend Biotech’s expectations for CARVYKTI™, such as Legend Biotech’s manufacturing and commercialization expectations for CARVYKTI™ and the potential effect of treatment with CARVYKTI™; statements about submissions for cilta-cel to, and the progress of such submissions with, the
|
|
||||
|
CONDENSED CONSOLIDATED STATEMENTS OF PROFIT OR LOSS |
||||
|
Three months ended |
||||
|
(in thousands, US$, except share and per share data) |
2022
|
|
2021
|
|
|
|
|
|
||
|
|
|
|
||
|
REVENUE |
40,827 |
|
13,682 |
|
|
Other income and gains |
1,012 |
|
722 |
|
|
Research and development expenses |
(81,346) |
|
(71,072) |
|
|
Administrative expenses |
(12,657) |
|
(8,742) |
|
|
Selling and distribution expenses |
(21,302) |
|
(13,417) |
|
|
Other expenses |
(1,527) |
|
(2,034) |
|
|
Fair value gain of warrant liability |
34,900 |
|
- |
|
|
Finance costs |
(994) |
|
(38) |
|
|
|
|
|
||
|
LOSS BEFORE TAX |
(41,087) |
|
(80,899) |
|
|
|
|
|
||
|
Income tax expense |
- |
|
- |
|
|
|
|
|
||
|
LOSS FOR THE PERIOD |
(41,087) |
|
(80,899) |
|
|
Attributable to: |
|
|
|
|
|
Ordinary equity holders of the parent |
(41,087) |
|
(80,899) |
|
|
|
|
|
||
|
Loss per share attributable to ordinary equity holders of the parent: |
|
|
|
|
|
Ordinary shares – basic |
(0.13) |
|
(0.30) |
|
|
Ordinary shares – diluted |
(0.13) |
|
(0.30) |
|
|
|
|
|
||
|
Shares used in loss per share computation: |
|
|
|
|
|
Ordinary shares – basic |
308,699,034 |
|
266,293,913 |
|
|
Ordinary shares – diluted |
308,699,034 |
|
266,293,913 |
|
|
|
||||
|
CONDENSED CONSOLIDATED STATEMENTS OF FINANCIAL POSITION |
||||
|
|
|
|||
|
(in thousands, US$) |
|
|
||
|
|
|
|
||
|
NON-CURRENT ASSETS |
|
|
||
|
Property, plant and equipment |
156,005 |
145,724 |
||
|
Advance payments for property, plant and equipment |
258 |
2,168 |
||
|
Right-of-use assets |
7,393 |
7,186 |
||
|
Other non-current assets |
4,912 |
5,148 |
||
|
Intangible assets |
4,517 |
4,684 |
||
|
Time deposits |
4,726 |
4,705 |
||
|
|
|
|
||
|
Total non-current assets |
177,811 |
169,615 |
||
|
|
|
|
||
|
CURRENT ASSETS |
|
|
||
|
Inventories |
2,895 |
1,749 |
||
|
Trade receivables |
50,451 |
50,410 |
||
|
Prepayments, other receivables and other assets |
16,651 |
12,754 |
||
|
Financial assets measured at amortized cost |
29,974 |
29,937 |
||
|
Financial assets at fair value through profit or loss |
99,995 |
- |
||
|
Pledged deposits |
1,448 |
1,444 |
||
|
Time deposits |
283,505 |
163,520 |
||
|
Cash and cash equivalents |
377,786 |
688,938 |
||
|
|
|
|
||
|
Total current assets |
862,705 |
948,752 |
||
|
|
|
|
||
|
Total assets |
1,040,516 |
1,118,367 |
||
|
|
|
|
||
|
CURRENT LIABILITIES |
|
|
||
|
Trade and notes payables |
9,712 |
7,043 |
||
|
Other payables and accruals |
96,055 |
123,464 |
||
|
Government grants |
320 |
304 |
||
|
Warrant liability |
53,000 |
87,900 |
||
|
Lease liabilities |
883 |
911 |
||
|
Contract liabilities |
65,560 |
60,644 |
||
|
|
|
|
||
|
Total current liabilities |
225,530 |
280,266 |
||
|
|
|
|
||
|
NON-CURRENT LIABILITIES |
|
|
||
|
Contract liabilities |
245,850 |
242,578 |
||
|
Lease liabilities |
1,630 |
1,593 |
||
|
Interest-bearing loans and borrowings |
126,714 |
120,462 |
||
|
Other non-current liabilities |
356 |
396 |
||
|
Government grants |
1,873 |
1,866 |
||
|
|
|
|
||
|
|
|
|||
|
(in thousands, US$) |
|
|
||
|
Total non-current liabilities |
376,423 |
366,895 |
||
|
|
|
|
||
|
Total liabilities |
601,953 |
647,161 |
||
|
|
|
|
||
|
EQUITY |
||||
|
Share capital |
31 |
31 |
||
|
Reserves |
438,532 |
471,175 |
||
|
|
|
|
||
|
Total ordinary shareholders’ equity |
438,563 |
471,206 |
||
|
|
|
|
||
|
Total equity |
438,563 |
471,206 |
||
|
|
|
|
||
|
Total liabilities and equity |
1,040,516 |
1,118,367 |
|
|
||||
|
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS |
||||
|
|
Three months ended |
|||
|
(in thousands, US$) |
2022
|
2021
|
||
|
|
|
|
||
|
LOSS BEFORE TAX |
(41,087) |
(80,899) |
||
|
|
|
|
||
|
CASH FLOWS USED IN OPERATING ACTIVITIES |
(78,687) |
(26,787) |
||
|
|
|
|
||
|
CASH FLOWS USED IN INVESTING ACTIVITIES |
(232,500) |
(17,150) |
||
|
|
|
|
||
|
CASH FLOWS FROM FINANCING ACTIVITIES |
25 |
207 |
||
|
|
|
|
||
|
|
(311,162) |
(43,730) |
||
|
|
|
|
||
|
Effect of foreign exchange rate changes, net |
10 |
337 |
||
|
Cash and cash equivalents at beginning of the period |
688,938 |
455,689 |
||
|
|
|
|
||
|
CASH AND CASH EQUIVALENTS AT END OF THE PERIOD |
377,786 |
412,296 |
||
|
|
|
|
||
|
ANALYSIS OF BALANCES OF CASH AND CASH EQUIVALENTS |
|
|
||
|
Cash and bank balances |
667,465 |
462,552 |
||
|
Less: Pledged deposits |
1,448 |
256 |
||
|
Time deposits |
288,231 |
50,000 |
||
|
Cash and cash equivalents as stated in the statement of financial position |
377,786 |
412,296 |
||
|
Cash and cash equivalents as stated in the statement of cash flows |
377,786 |
412,296 |
||
____________________
1 CARVYKTI™ Prescribing Information.
2 CARVYKTI™ (ciltacabtagene autoleucel), BCMA-Directed CAR-T Therapy, Receives
3 CARVYKTI® (ciltacabtagene autoleucel) Granted Conditional Approval by the
4
5
6
7
8 Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5),548-567. doi:10.1002/ajh.25791.
9 Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443- 2448.
10 Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38- targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266-2275.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220601005384/en/
Investor:
joanne.choi@legendbiotech.com
crystal.chen@legendbiotech.com
Press:
tina.carter@legendbiotech.com
(908) 331-5025
Source:



